OUR
SERVICES
Qualified Person (QP) Services and Batch Release
Medelligent Pharmaceuticals Ltd (Medelligent) undertakes the certification and release of batches manufactured in the EU and/or in third countries. Our highly experienced Qualified Persons (QPs) cover non-sterile and sterile Finished Dosage Forms (FDFs) as well as starting materials including Active Pharmaceutical Ingredients (APIs). Access to EU-based laboratories which can undertake the testing of FDFs imported in the EU is also provided. Our QPs can undertake to establish Manufacturing/Import Authorization (MIA) as well as Wholesale Distribution Authorization (WDA) complying with EU Good manufacturing Practices (GMP). Consultancy can be offered on QP release challenges.

GMP Audits of API and FDF Manufacturers
Medelligent Pharmaceuticals Ltd (Medelligent) undertakes the certification and release of batches manufactured in the EU and/or in third countries. Our highly experienced Qualified Persons (QPs) cover non-sterile and sterile Finished Dosage Forms (FDFs) as well as starting materials including Active Pharmaceutical Ingredients (APIs). Access to EU-based laboratories which can undertake the testing of FDFs imported in the EU is also provided. Our QPs can undertake to establish Manufacturing/Import Authorization (MIA) as well as Wholesale Distribution Authorization (WDA) complying with EU Good manufacturing Practices (GMP). Consultancy can be offered on QP release challenges.
Quality Management Systems
Medelligent Pharmaceuticals Ltd (Medelligent) undertakes the certification and release of batches manufactured in the EU and/or in third countries. Our highly experienced Qualified Persons (QPs) cover non-sterile and sterile Finished Dosage Forms (FDFs) as well as starting materials including Active Pharmaceutical Ingredients (APIs). Access to EU-based laboratories which can undertake the testing of FDFs imported in the EU is also provided. Our QPs can undertake to establish Manufacturing/Import Authorization (MIA) as well as Wholesale Distribution Authorization (WDA) complying with EU Good manufacturing Practices (GMP). Consultancy can be offered on QP release challenges.
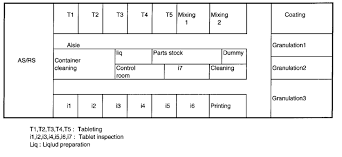
Plant Layout Materials and Machinery Selection
Medelligent Pharmaceuticals Ltd (Medelligent) undertakes the certification and release of batches manufactured in the EU and/or in third countries. Our highly experienced Qualified Persons (QPs) cover non-sterile and sterile Finished Dosage Forms (FDFs) as well as starting materials including Active Pharmaceutical Ingredients (APIs). Access to EU-based laboratories which can undertake the testing of FDFs imported in the EU is also provided. Our QPs can undertake to establish Manufacturing/Import Authorization (MIA) as well as Wholesale Distribution Authorization (WDA) complying with EU Good manufacturing Practices (GMP). Consultancy can be offered on QP release challenges.
Project Management
Medelligent Pharmaceuticals Ltd (Medelligent) undertakes the certification and release of batches manufactured in the EU and/or in third countries. Our highly experienced Qualified Persons (QPs) cover non-sterile and sterile Finished Dosage Forms (FDFs) as well as starting materials including Active Pharmaceutical Ingredients (APIs). Access to EU-based laboratories which can undertake the testing of FDFs imported in the EU is also provided. Our QPs can undertake to establish Manufacturing/Import Authorization (MIA) as well as Wholesale Distribution Authorization (WDA) complying with EU Good manufacturing Practices (GMP). Consultancy can be offered on QP release challenges.

