Elevate Your Pharmaceutical Compliance.
Discover tailored consulting services designed to enhance your pharmaceutical operations and ensure compliance with industry and legal requirements.
Qualified Person (QP) Services and Batch Certification / Release and QP Declaration for API
 Medelligent Pharmaceuticals Ltd (Medelligent) undertakes the certification and release of batches manufactured in the EU and/or third countries. Our highly experienced Qualified Persons perform batch certification and release for non-sterile and sterile Finished Dosage Forms (FDFs). Access to EU-based laboratories for testing of FDFs imported into the EU is also provided. Expert advice can be offered on QP release and quality challenges. Release of herbal medicinal products batches is also undertaken by Medelligent.
Medelligent Pharmaceuticals Ltd (Medelligent) undertakes the certification and release of batches manufactured in the EU and/or third countries. Our highly experienced Qualified Persons perform batch certification and release for non-sterile and sterile Finished Dosage Forms (FDFs). Access to EU-based laboratories for testing of FDFs imported into the EU is also provided. Expert advice can be offered on QP release and quality challenges. Release of herbal medicinal products batches is also undertaken by Medelligent.
Qualification and Validation
 We undertake Qualification of complex equipment and systems such as BMS/EMS, HVAC, and Validation of operations such as Cleaning Validation, Process Validation. We carry out FAT and SAT on equipment to safeguard our customers’ interests.
We undertake Qualification of complex equipment and systems such as BMS/EMS, HVAC, and Validation of operations such as Cleaning Validation, Process Validation. We carry out FAT and SAT on equipment to safeguard our customers’ interests.
Risk Assessments and Other Activities
 Our team has the expertise to carry out risk assessments for: GxP operations (GMP/GDP), excipients, temperature excursions, OOS, Business Continuity Plans, nitrosamines etc. We are familiar with several RA Tools. Our dedicated team can draft qualification protocols (URS, IQ, OQ/PQ) and reports, thermal mapping studies plan and report, Product Quality Reviews that include statistical parameters, Technical Agreements, Management Reviews and anything else needed to develop and improve our customers' QMS. Besides our GxP expertise, we can build QMS for ISO such as 9001:2015.
Our team has the expertise to carry out risk assessments for: GxP operations (GMP/GDP), excipients, temperature excursions, OOS, Business Continuity Plans, nitrosamines etc. We are familiar with several RA Tools. Our dedicated team can draft qualification protocols (URS, IQ, OQ/PQ) and reports, thermal mapping studies plan and report, Product Quality Reviews that include statistical parameters, Technical Agreements, Management Reviews and anything else needed to develop and improve our customers' QMS. Besides our GxP expertise, we can build QMS for ISO such as 9001:2015.
Quality Management Systems
 Medelligent undertakes the development or upgrading of Quality Management Systems suitable for pharmaceutical companies that require Manufacturing/Import Authorization (MIA), Wholesale Distribution Authorization (WDA), or ISO Certification, in compliance with EU Good Manufacturing Practices (GMP), EU Good Distribution Practices (GDP), and ISO standards.
Medelligent undertakes the development or upgrading of Quality Management Systems suitable for pharmaceutical companies that require Manufacturing/Import Authorization (MIA), Wholesale Distribution Authorization (WDA), or ISO Certification, in compliance with EU Good Manufacturing Practices (GMP), EU Good Distribution Practices (GDP), and ISO standards.
Plant Layout, Selection of Materials and Machinery
 We can design layouts or propose layout modifications of new or existing FDF manufacturing or packaging facilities. Our layout proposal is based on customer needs and requested capacities. We are committed to deliver a cGMP/cGDP conceptual design to meet customer requirements. Materials and machinery are selected from reliable and price competitive suppliers from around the world. Consulting on system specifications, such as HVAC, as well as the selection of materials and machinery is offered. Warehouse design according to cGDP principles is also an integral part of our activities.
We can design layouts or propose layout modifications of new or existing FDF manufacturing or packaging facilities. Our layout proposal is based on customer needs and requested capacities. We are committed to deliver a cGMP/cGDP conceptual design to meet customer requirements. Materials and machinery are selected from reliable and price competitive suppliers from around the world. Consulting on system specifications, such as HVAC, as well as the selection of materials and machinery is offered. Warehouse design according to cGDP principles is also an integral part of our activities.
Pharmacovigilance
 We offer local pharmacovigilance services, including the appointment of a local QPPV for Cyprus, and assistance with the required Pharmacovigilance Quality Management System.
We offer local pharmacovigilance services, including the appointment of a local QPPV for Cyprus, and assistance with the required Pharmacovigilance Quality Management System.
Audits
 Our team performs GMP audits of manufacturers of sterile and non-sterile FDFs, APIs, starting materials, and packaging materials, as well as GDP audits of wholesalers and ISO standard audits. We have conducted more than 100 audits, including audits of biotechnology product manufacturers, FDF sterile product manufacturers, vaccine manufacturers, and API manufacturers.
Our team performs GMP audits of manufacturers of sterile and non-sterile FDFs, APIs, starting materials, and packaging materials, as well as GDP audits of wholesalers and ISO standard audits. We have conducted more than 100 audits, including audits of biotechnology product manufacturers, FDF sterile product manufacturers, vaccine manufacturers, and API manufacturers.
Project Management
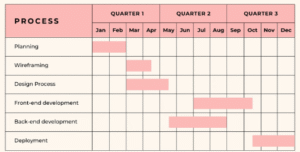 Medelligent manages projects from ground zero all the way to obtaining EU certification. Our team can undertake projects from the design stage of premises to the development and implementation of the Quality Management System, and subsequently EU GMP/GDP/ISO certification.
Medelligent manages projects from ground zero all the way to obtaining EU certification. Our team can undertake projects from the design stage of premises to the development and implementation of the Quality Management System, and subsequently EU GMP/GDP/ISO certification.
Herbal Medicinal Products
 Medelligent can undertake the establishment Quality of an EU GMP Management System, equipment selection, and advice on herbal medicinal manufacturing. Advice can also be offered on GACP (Good Agricultural and Collection Practices).
Medelligent can undertake the establishment Quality of an EU GMP Management System, equipment selection, and advice on herbal medicinal manufacturing. Advice can also be offered on GACP (Good Agricultural and Collection Practices).
Training
 Medelligent performs GMP and GDP training for companies and institutions in Cyprus and abroad. Training is also offered on specialized topics related to the pharmaceutical industry.
Medelligent performs GMP and GDP training for companies and institutions in Cyprus and abroad. Training is also offered on specialized topics related to the pharmaceutical industry.
