Empowering Pharmaceutical Excellence
Leading the way in GMP & GDP Compliance
Discover Medelligent’s commitment in advancing pharmaceutical systems through expert consulting services.
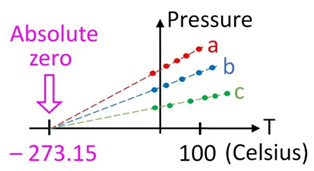
The outcome of human activities is uncertain. In pharmaceuticals there is an inherent risk of errors. We go beyond regulations by understanding regulations from their scientific routes. However, with advanced knowledge, risk assessment, and the use of appropriate tools, we consult our customers to reduce this inherent uncertainty. We cannot reach perfection encountered at absolute zero, but “We can reduce uncertainty by employing Risk Assessment Tools, Knowledge and Rational Thinking”
Perfection is virtually unattainable because the laws of physics stipulate so.
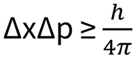
Our Core Competencies
Qualified Person (QP) Services and Batch Certification / Release and QP Declaration for API
Qualification and Validation
Risk assesment and other activities
Quality Management Systems
Plant Layout, Selection of Materials and Machinery
Pharmacovigilance
Audits
Project Management
Herbal medicinal products
Training
%
Ensuring the product reaches market as intended
%
Maximising confidence by
Minimising risk to acceptable levels.
Medelligent brings over 25 years of pharmaceutical expertise to deliver tailored consulting services for the industry. We specialize in guiding companies through the evolving demands of Good Manufacturing Practice (GMP) and good distiribution practicse (GDP) compliance with expert insights, training, and hands-on support.
Our team includes experienced EU Qualified Persons (QPs), Responsible Persons (RPs), and subject matter experts, all dedicated to helping your business achieve compliance with practical, timely, and effective solutions.
Grounded in deep knowledge and pharmaceutical technology principles, our consulting approach ensures that every solution we provide is both scientifically sound and business-ready.
